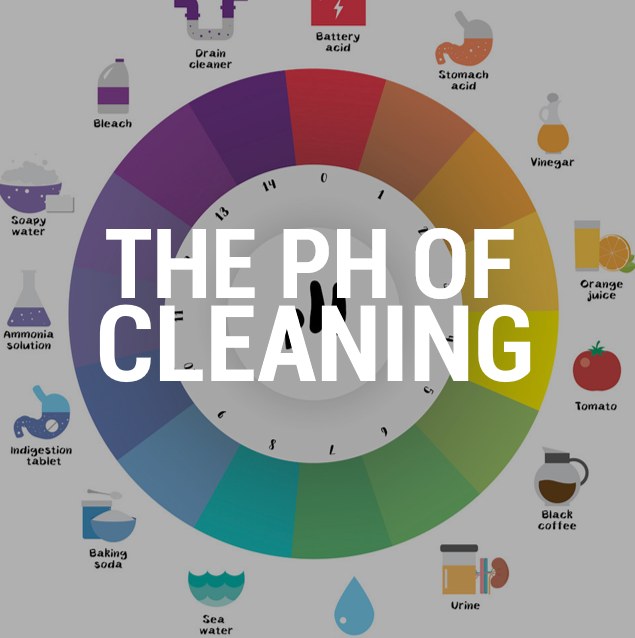
What is pH, anyway?
pH stands for ‘potential hydrogen’, and is a measure of the acidity or alkalinity of a given chemical when mixed with water. A solution with a pH level of 0 will be very acidic (a high concentration of hydrogen ions); a solution with a pH level of 7 will be neutral (equal concentration of hydrogen ions and hydroxy ions, like water); and a solution with a pH level of 14 (a high concentration of hydroxy ions) is considered alkaline.
Many people know that something highly acidic (like battery acid) is corrosive, but what’s less well understood is that at the other end of the scale, highly alkaline substances (like bleach) are also toxic.
What does this mean for cleaning?
Generally speaking, effective cleaning products tend to be found at either end of the pH scale: Soapy water and bleach are highly alkaline, while vinegar is an acid – all of these can be effective cleaning products, depending on the soils, dirt and composition of the surfaces to be cleaned.
The higher the pH level, the more corrosive a cleaner will be. If the substance you want to remove is acidic, you’ll generally want to use an alkaline cleaner; if the substance you want to remove is alkaline, you’ll generally want to use an acidic cleaner. Both will help to bring the substance to a neutral pH, which will facilitate removal.
It’s important to remember that while very high and very low pH cleaners can be very effective, they also carry risks: The chemical reactions they can create mean that surfaces can be damaged, toxic fumes can be released, and users can suffer skin damage if proper protection isn’t used. More neutral solutions (with a pH of 5-9) can be safer – but sometimes not as effective.
Acidic cleaners
These are cleaners with mineral acids/chelates as the active ingredient. Acidic cleaners tend to be found in bathroom cleaners because they can remove calcium buildup and scaly deposits.
Alkaline cleaners
These cleaners, which include ammonia, are generally used to dissolve soils that are based in fats, oils and proteins. Alkaline cleaners break down these fats and oils and make them easier to remove.
Neutral cleaners
With a pH level of 5-9, neutral cleaners tend to be milder on both the surfaces to be cleaned and the soils that are to be removed. This also means they’re generally less toxic and dangerous – baking soda, with a pH of 9, for example, is often recommended as a ‘safe’ or ‘natural’ cleaner. However, neutral cleaners are typically less effective than acidic or alkaline ones.
What kind of cleaner should you use?
Before you figure out which pH level your cleaner should have, you’ll want to determine two things: The surface you’re cleaning and the substances you’re trying to remove.